Elevate your local knowledge
Sign up for the iNFOnews newsletter today!
Sign up for the iNFOnews newsletter today!
Selecting your primary region ensures you get the stories that matter to you first.
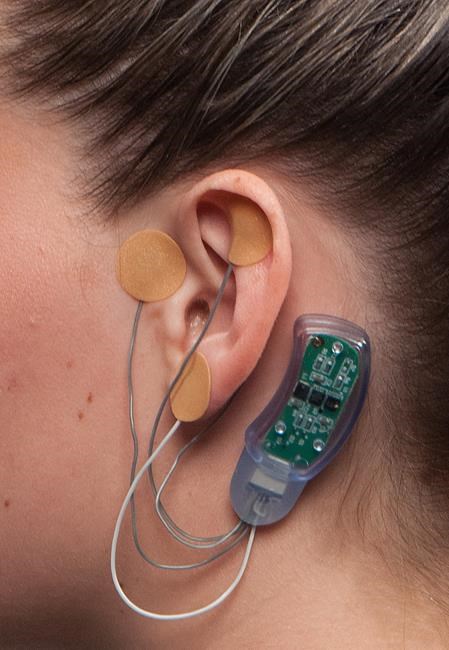
WASHINGTON – U.S. health authorities have cleared a brain-stimulating device for patients suffering from debilitating withdrawal symptoms caused by addiction to heroin and other opioids.
The nerve stimulator is the first device to treat such symptoms including joint pain, anxiety, stomach aches and insomnia. The announcement Wednesday by the Food and Drug Administration comes amid an epidemic of opioid abuse, which includes both illegal narcotics like heroin and prescription painkillers.
The device, known as NSS-2 Bridge, is worn behind the ear where several electrodes stimulate nerves in the brain and spinal cord to relieve symptoms. The FDA says a study of more than 70 patients showed a 30 per cent decrease in symptoms within 30 minutes of using the device.
Nerve stimulators have previously been approved to treat epilepsy and depression.
News from © iNFOnews.ca, . All rights reserved.
This material may not be published, broadcast, rewritten or redistributed.

This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Want to share your thoughts, add context, or connect with others in your community?
You must be logged in to post a comment.