Gynecology group urges women to use backup contraception if took faulty ‘Pill’
TORONTO – The Society of Obstetricians and Gynecologists of Canada says women who may have taken birth control pills from faulty packs should use a backup form of contraception for the time being.
A senior official of the organization says women may also need to talk with their doctors about taking a pregnancy test.
The advice is prompted by a major birth control recall involving the drug Alysena 28.
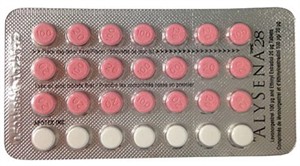
A batch of about 50,000 faulty packets of the drug were distributed in Canada; the packages contained two weeks’ worth of sugar pills, not one.
Dr. Jennifer Blake of the Society of Obstetricians and Gynecologists of Canada says missing a week of the drug could result in pregnancies.
She says Plan B, the so-called morning after pill, would be an option for women who have had what amounts to unprotected sex in the last three to five days.
Women taking birth control pills swallow the drugs for 21 days each menstrual cycle. Because of the risk that they might not remember to resume taking their pills at the right time, many of these contraceptives are packed with a pill for each of the 28 days in a cycle — 21 are drug and seven are placebos.
But with these faulty packs of Alysena, there were two weeks of white placebos and two weeks of pink contraceptives.
Blake, the CEO of the society, suggested the turn of event was upsetting, because Canada has had a variety of safe and reliable birth control options for women.
“Certainly women using it (Alysena 28) right now should be advised to use an additional method of protection, because they wouldn’t have the appropriate level of protection from the faulty packages,” she says.
The drug, marketed by the drug firm Apotex, is a generic version of the most popular birth control pill in use, a drug called Alesse.
Alesse is not involved in this recall, Blake stresses. In fact, the only drug implicated is Alysena 28.
Some prescription drug plans require pharmacists to replace higher-priced brand name drugs with generic versions if a generic is available. That may make it difficult to get a sense of who was on this drug, Blake says. In fact, she noted, women themselves may not be clear.
“The generic chose a very similar name and look to the leading birth control pill. And that does create confusion in everyone’s mind,” she says.
Apotex is recalling about 50,000 packets in a lot of drugs that was distributed between Dec. 4 and 17, 2012, says Elie Betito, the company’s director of public and governmental affairs. He says seven other batches have been released since the faulty batch was pushed into the system.
Betito says the company is working with the manufacturer, Leon Farma of Spain, to try to figure out what went wrong.
Join the Conversation!
Want to share your thoughts, add context, or connect with others in your community? Create a free account to comment on stories, ask questions, and join meaningful discussions on our new site.




















Leave a Reply
You must be logged in to post a comment.