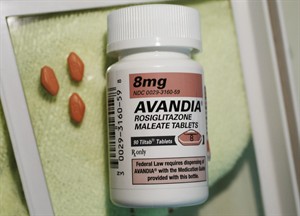
FDA overturns safety restrictions on diabetes pill Avandia based on new safety data
WASHINGTON – The Food and Drug Administration is lifting severe safety restrictions on the former blockbuster diabetes pill Avandia, citing recent data suggesting that the much-debated medication does not increase the risk of heart attack.
The repeal means patients will no longer have to enrol in a special registry to be eligible to receive the drug. That safety requirement, put in place in 2010, sharply reduced Avandia prescriptions in the U.S.
The ruling is a belated victory for British drugmaker GlaxoSmithKline after more than a half-decade defending the safety of Avandia, which was once the bestselling diabetes drug in the world.
Sales began plummeting in 2007 after researchers first raised questions about possible links to heart attacks. After three years of debate, the FDA limited access to the drug in 2010.
Join the Conversation!
Want to share your thoughts, add context, or connect with others in your community? Create a free account to comment on stories, ask questions, and join meaningful discussions on our new site.












Leave a Reply
You must be logged in to post a comment.