Elevate your local knowledge
Sign up for the iNFOnews newsletter today!
Sign up for the iNFOnews newsletter today!
Selecting your primary region ensures you get the stories that matter to you first.

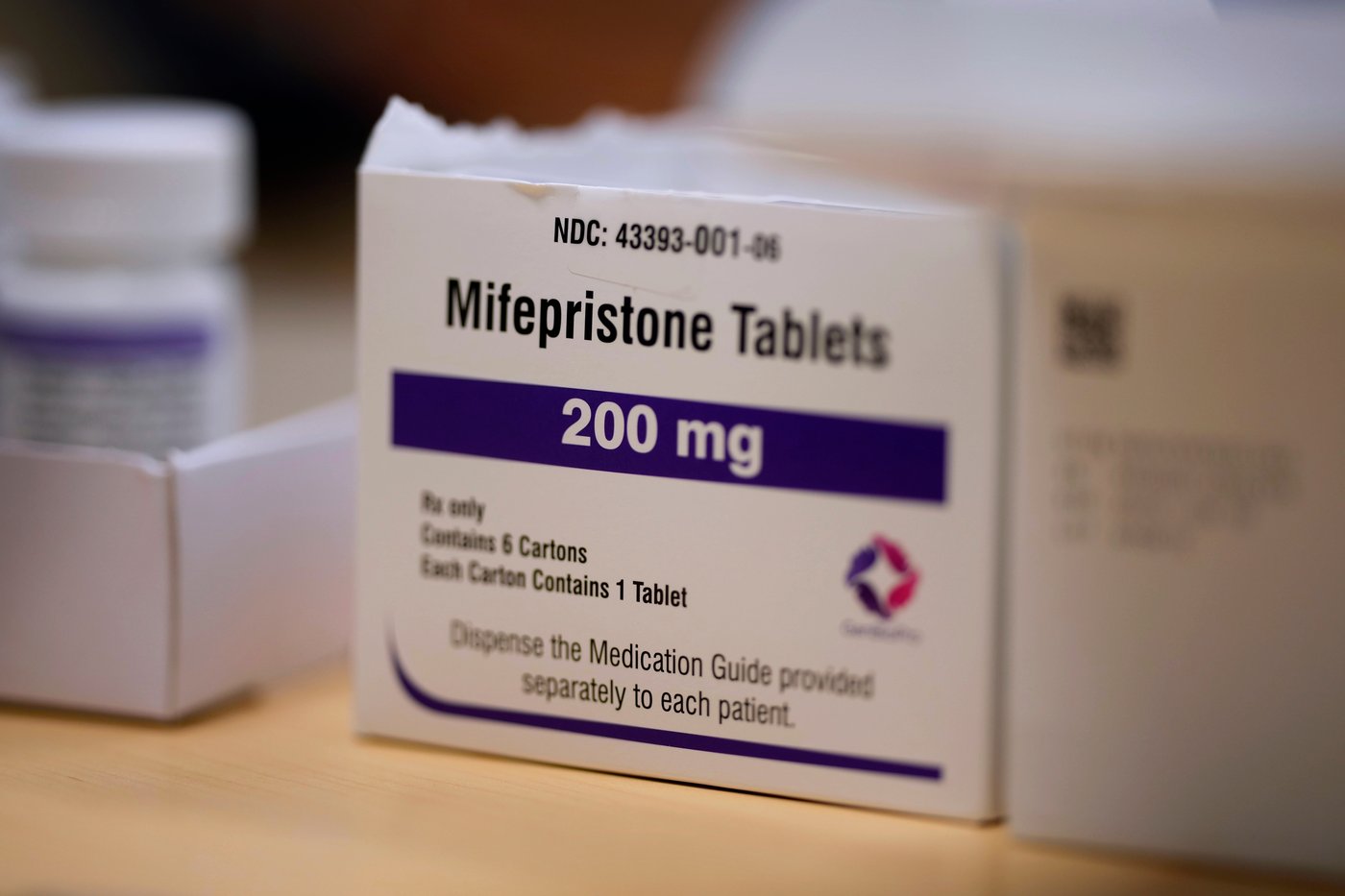
HONOLULU (AP) — The U.S. Food and Drug Administration violated the law by imposing restrictions on accessing mifepristone, a medication for abortions and miscarriage management, a federal judge in Hawaii ruled Thursday.
A lawsuit by the American Civil Liberties Union argues the FDA continues to overly restrict access to a safe medication without scientific justification. ACLU lawyers asked the judge to find that the FDA violated the law but didn’t seek an immediate elimination of the restrictions, which currently include special certification for prescribers and pharmacies and requiring patients to review a counseling form.
The FDA’s 2023 decision to maintain the restrictions was unlawful under the Administrative Procedure Act, “by failing to provide a reasoned explanation for its restrictive treatment of the drug,” U.S. District Judge Jill Otake’s ruling says.
Otake’s ruling instructs the FDA to consider relevant evidence the agency allegedly disregarded. In the meantime, the restrictions remain in place.
The decision comes as the pill used in most U.S. abortions continues to be ensnared in politics that have plagued it for nearly a decade, with many wondering if it will be further restricted under President Donald Trump’s Republican administration. Trump’s top health officials, including Health Secretary Robert F. Kennedy Jr., face growing pressure from abortion opponents to reevaluate mifepristone, which was approved 25 years ago and has repeatedly been deemed safe and effective by FDA scientists.
The case dates to 2017 and has spanned both Republican and Democratic administrations.
“Today’s decision is a victory for everyone who believes that our access to safe and essential medicines should be dictated by science, not politics,” Julia Kaye, senior staff attorney with the ACLU Reproductive Freedom Project, said in a statement. “Despite decades of real-world experience and mountains of evidence proving mifepristone’s safety, the FDA regulates this medication more heavily than 99 percent of prescription drugs.”
When the case first started, a key restriction required patients to pick up the medication in person at a hospital, clinic or medical office. That restriction was eventually removed and the pill can be sent through the mail. The lawsuit continues to focus on the remaining restrictions that the ACLU says disproportionately impact patients who already face difficulties accessing healthcare, such as those who are low-income or live in rural areas.
Justice Department attorneys involved in the case didn’t immediately respond to an email from The Associated Press seeking comment on the ruling. They have argued previously the FDA has already reduced the burden by removing the in-person dispensing requirement.
Hawaii law allows abortion until a fetus would be viable outside the womb. After that, it’s legal if a patient’s life or health is in danger. The state legalized abortion in 1970, when it became the first in the nation to allow the procedure at a woman’s request.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Want to share your thoughts, add context, or connect with others in your community?
You must be logged in to post a comment.